https://www.freepressjournal.in/business/clinical-trials-for-ayurvedic-cure-of-covid-19-are-underway-says-patanjalis-acharya-balkrishna
An ayurvedic cure for Covid-19 is underway. Expect more announcements — Acharya Balkrishna.
RN Bhaskar, June 1, 2020
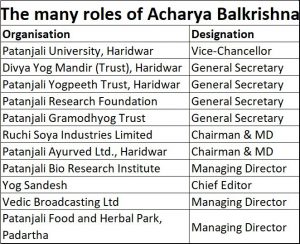
 Most people recognise Acharya Balkrishna as an associate of Patanjali. But few know that he is much more than that. He is, among other things, a great bhakt (spiritual follower) of the tradition of yoga & ayurveda in India. He promotes ancient healing and lifestyle traditions discovered by ancient sages in this country. He is founder secretary of Patanjali Trust, Patanjali Research – and these are just two of the many designations he holds in the sprawling Patanjali group of institutions (see chart). The group currently accounts for direct employment of over 50,000 people.
Most people recognise Acharya Balkrishna as an associate of Patanjali. But few know that he is much more than that. He is, among other things, a great bhakt (spiritual follower) of the tradition of yoga & ayurveda in India. He promotes ancient healing and lifestyle traditions discovered by ancient sages in this country. He is founder secretary of Patanjali Trust, Patanjali Research – and these are just two of the many designations he holds in the sprawling Patanjali group of institutions (see chart). The group currently accounts for direct employment of over 50,000 people.
Many call him a great visionary, highly ascetic, energetic, diligent, simple, easy-going and a versatile personality with multi- dimensional skills who is constantly engaged in the service of mankind. He is a great scholar of ayurveda, the Sanskrit language and the Vedas. He has published more than 130 Research Articles in various national and International Journals and has got 41 Patents/rights.He has aAuthored more than 120 books on Yoga and Ayurveda and edited more than 20 unpublished ancient Ayurveda manuscrips.
But for the past few months, he is focussed on finding a cure and a vaccine for the dreaded coronavirus. Clinical trials are now underway under Jaipur University, and human trials based on scientific evidence are in progress. Acharyaji is confident that the Patanjali group will soon come out with findings that could stun the world.
He spoke with RN Bhaskar, of Free Press Journal, in an exclusive interview, of his efforts in the battle against Covid-19.
Given below are edited excerpts:
 Could you let me know what steps you have been taking in the areas related to Covid-19?
Could you let me know what steps you have been taking in the areas related to Covid-19?
To combat COVID-19 virus with Ayurveda, we have screened close to 1,000 phytochemicals from more than 100 medicinal plants, in-sillico [of scientific experiments or research conducted or produced by means of computer modelling or computer simulation]. We have already developed the required protocols and are now proceeding with evidence based medicines for its cure and treatment. Please note, that we are not talking about preventing Covid-19 through building of immunity. We are talking about a cure through treatment.
We looked for their binding affinities to COVID-19, essential proteins and host protein interactions. We have discovered that natural phytochemicals in Ashwagandha, Giloy (an ayurvedic herb) and Tulsi indeed have the potential to combat COVID-19 and its pathogenicity.
Our data, for the first time, show that natural phytochemicals could well be the viable options for controlling COVID-19 entry into host cells, and Ashwagandha may be the first choice of herbs in these directions to curb the COVID-19 infectivity. This work is currently under Peer-Review for publication in Virology Journal, in Springer-Nature, and available at their pre-print server (https://www.researchsquare.com/article/rs-17806/v1).
What is the treatment process that you plan?
The proposed treatment regime is designed to add efficacy and potency of Ayurvedic medicines in treating COVID-19 infection in combination with ICMR (Indian Council of Medical Research) approved hydroxyl-chloroquinone. This regime is meticulously designed with clinical relevance for the ayurvedic intervention therapy on war footing to cope with COVID-19 infection.
You may recall that the ICMR had recently — on March 26, 2020 — approved the use of hydroxyl-chloroquinone for prophylactic treatment of COVID-19 infection.
Chinese traditional medicines have been used in combination with allopathic treatments for symptomatic alleviation in COVID-19 cases. Ayurveda, one of the world renowned forms of Indian traditional medicine, mentions several immunity boosting therapeutics. This is what we propose to bring to the fore-front in healing COVID-19 ailments, concurrent with ICMR prescribed allopathic treatments.
Today we have three NABL approved laboratories (National Accreditation Board for testing and Calibration Laboratories — https://www.nabl-india.org/nabl/index.php?c=searchlab&m=index&Itemid=177 – we have the largest research facilities from among all Ayurveda related institutes and organisations in India. One of them is dedicated to research into Covid-19. Collectively, we have over 500 researchers working with us, of which 100 are post-doctoral researchers.
But was the path to doing clinical trials easy?
It was tough.
We had already developed a treatment protocol by the end of March. But it needed government approval. For that we needed to be certified.
We told the government that many Covid-19 patients had become negative after consuming our course of treatment. But to get approval for our medicines as a standard cure for Covid-19, we now wanted permission to conduct clinical trials, for which a set of clearances are required from the government.
Such clearances would enable us to prepare clinical evidence and evidence based trials that are required . We kept trying repeatedly for such clearances. But since such procedures were easy for allopathy processes, we could not even get an entry with ICMR..
However, our efforts finally bore fruit. We got registered under CTRI (Clinical Trials Regulator of India – http://ctri.nic.in/Clinicaltrials/login.php) and began our clinical trials atone of the departments under Jaipur University.
Thus, while we have already successfully treated thousands of patients who were suffering from Covid-19, the real work of proving this efficacy through clinical trials has just begun. We are now adopting the right processes required, and the first trials have been encouraging with excellent results. We hope to publish our findings shortly.
What are the other cures you are working on?
There are several. Of them some are worth mentioning.
We have been working on cures for cancer and diabetes. Unfortunately, no authentic medicine has been prepared so far due to the absence of clinical trials of ayurvedic medicines. This is despite the fact that thousands of cancer patients have got benefited at Patanjali ayurveda hospital.
As regards diabetes, blood sugar is easily controlled through ayurveda. So it is with Dengue. Giloya juice is effective in its control. Dengunil made of papaya leaf juice, tulsi and giloya is also beneficial in this area.
But the same absence of clinical trials is the stumbling block
With our CTRI accreditation, and our clinical trials in respect of Covid-19 treatment, we think you will see other clinical trials also taking place one after another.
As I mentioned earlier, Ashwagandha might become one of the potent ingredients against COVID-19. Our findings are under peer-review in Virology Journal, in Springer-Nature, and available at their preprint server.
============
Box item
An excerpt from the documentation on the cure for Covid-19
For systemic symptomatic treatment of COVID-19, poly-herbo-mineral formulation, Tablet Swasari Ras has been generated for providing relief in lung congestion and to reduce mucus accumulation in the lungs. We have several thousand patient’s clinical data in Patanjali Electronic Medical Record (PEMR) system.
We have also validated Swasari Ras in our pre-clinical models of lung diseases. Treatment of Swasari Ras reduced excessive mucus production and airway remodelling; decreased pro-inflammatory cytokines in the inflamed lungs; and kept healthy oxidation state of the lungs.
This work has been recently published in Biomedicine and Pharmacotherapy, an Elsevier publication [47] [https://doi.org/10.21203/rs.3.rs-17806/v1].








































COMMENTS